Genvida - Solidstate nanogap sequencing
This week I’m planning to look at a few different Chinese nanopore sequencing startups. There are in fact, a surprising number of them. I’ve previously talked about AxBio, but I’m aware of at least 3 more.
Today I’m looking at tunneling current startup, Genvida. Genvida is based in Hong Kong, and have raised an undisclosed series A.
There’s a surprisingly interesting video on youtube. Not really for what they say, but for the equipment shown. In particular, you can see they’re using the Axopatch 200B, this has been featured in many classic nanopore papers including the foundational work on MspA. It’s a single channel instrument, suggesting that they are currently taking measurements from single pores:
Later on in the video we can see a small fluidic cell. This was kind of exciting for me to see, as I have one of these in my backroom! It’s a fluidic cell from Nanopore Solutions (also sold via Zimmerman-Peacock). It’s actually a really nice fixture (if you have such a need I recommend reaching out to them):
Overall, the videos suggest that Genvida is just getting started, and using equipment that is commercially available. Elsewhere they show complete instruments, but I’d guess these are likely mockups:
They look nice though!
But what is Genvida actually proposing? Earlier this year, Genvida published a paper simulating a tunneling current approach. I went ahead and paid the horrendous fee to get a copy of this (hint, you can always support by subscribing).
The paper describes the basic nanogap sequencing approach. Genvida isn’t the first company to propose such an approach, and I was previously CTO of another tunneling current company (Quantum Biosystems).
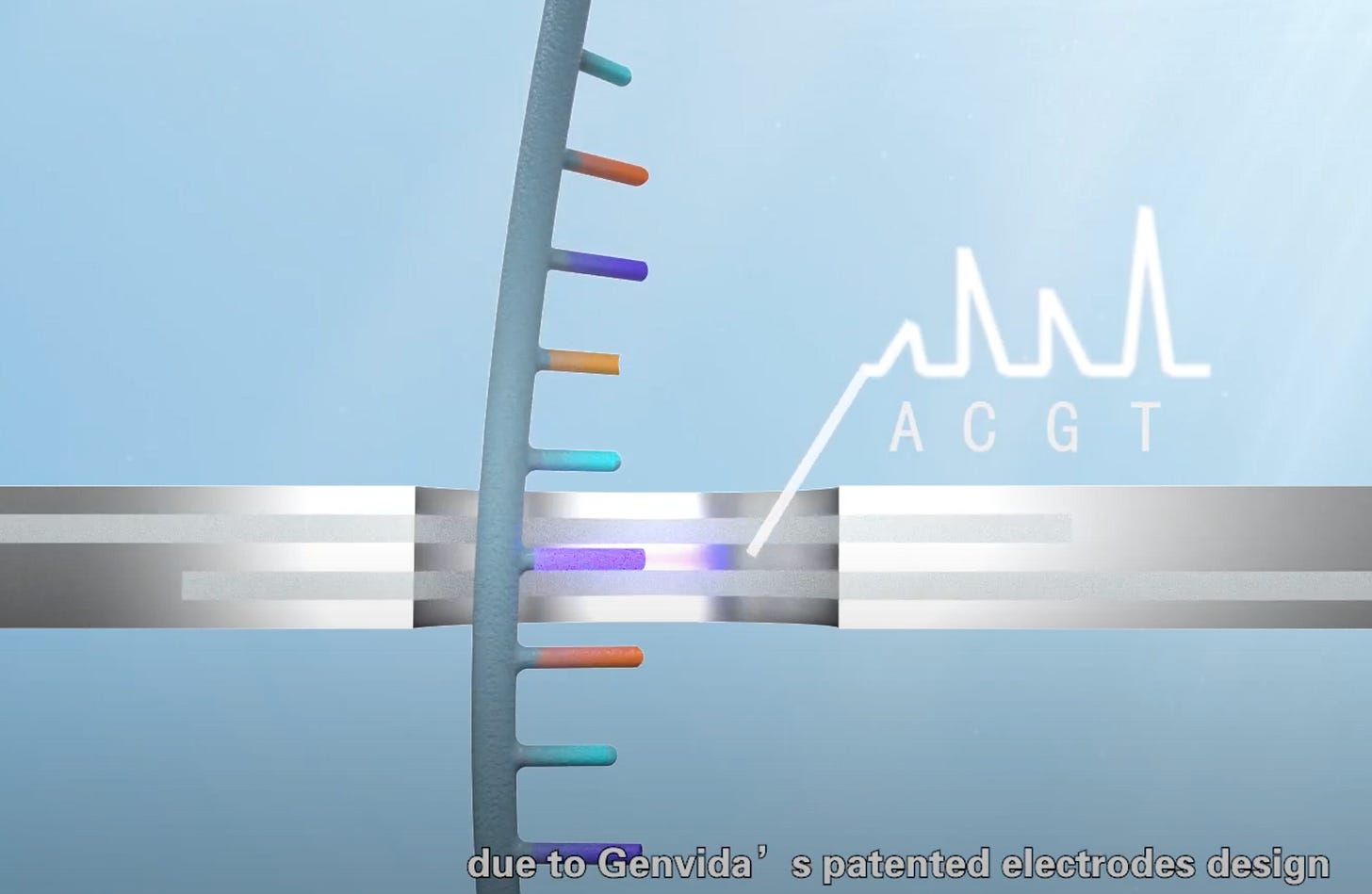
The approach is theoretically simple. Two electrodes, are placed a small distance (<1nm) apart. A bias voltage is put across the electrodes and the current measured. At such small distances electrons can “jump” across the gap, not by classically conducting, but through electron tunneling.
The trick then is that anything else that translocates (goes through) the gap will interfere with the tunneling current. This can be detected. In DNA sequencing the idea is that each nucleotide will somehow characteristically interfere with the tunneling current.
The paper shows simulations of single nucleobases in a nanogap, measuring the associated tunneling currents with the base in different orientations.
They first figure out which orientations are most likely to occur, by simulating the relative energies of each possible orientation:
With this in hand, they then calculate the average currents for each base. They use 3 different types of nanogaps. A pure gold (Gap-Au) , gold decorated with sulfur (Gap-AuS), and version of Gap-AuS which is ~0.1nm bigger.
This results in the following table of average currents:
This table is interesting. The pure gold gap doesn’t look great. But clearly we’re seeing an order of magnitude difference between nucleobases in Gap-AuS. Compared to protein nanopores these are very nicely separated. Our nucleobases are at 1100pA, 367pA 39pA and 2pA. In comparison to this, a protein pore like MspA, will give a total spread of ~20pA (at a similar 100mV bias).
But it’s not all good news. Take a look at the Gap-AuS and Gap-AuS′ results. In Gap-AuS C is at 367pA in Gap-AuS′ 3pA. That’s a huge difference for a ~0.1nm change in gap size. Large changes are somewhat expected as tunneling currents drop exponentially with distance. But as I understand it 0.1nm is less than the lattice stacking distance in gold… we’ll see why this might be important later…
They also graphically plot these as ratios:
As far as the paper goes the take home message appears to be that gold electrodes alone don’t look great, but decorated with sulfur look pretty good.
Looking at some of their other videos we can get a better sense of what they might be doing:
From the screen shot above it seems likely that they proposing the electrodes be embedded in the substrate. This makes a lot of sense. Currently it’s not really practical to fabricate electrodes that are separated by single digit nanometers. But you have much better control over the thickness of layers on a substrate. So, put one electrode on one layer, then a 1nm insulating layer, and the second electrode above the first.
Great! But then how much control do we have over the electrode distance? We saw above that a 0.1nm change in the gap size resulted in dramatically different behaviour. As far as I can tell, layers of silicon or gold would be thicker than this. And controlling the layer thickness so precisely seems like a hard problem.
Not only this, but if the electrodes are overlapping as show above, you would get tunneling through the insulating layer. This is is going to add additional noise, making detection harder.
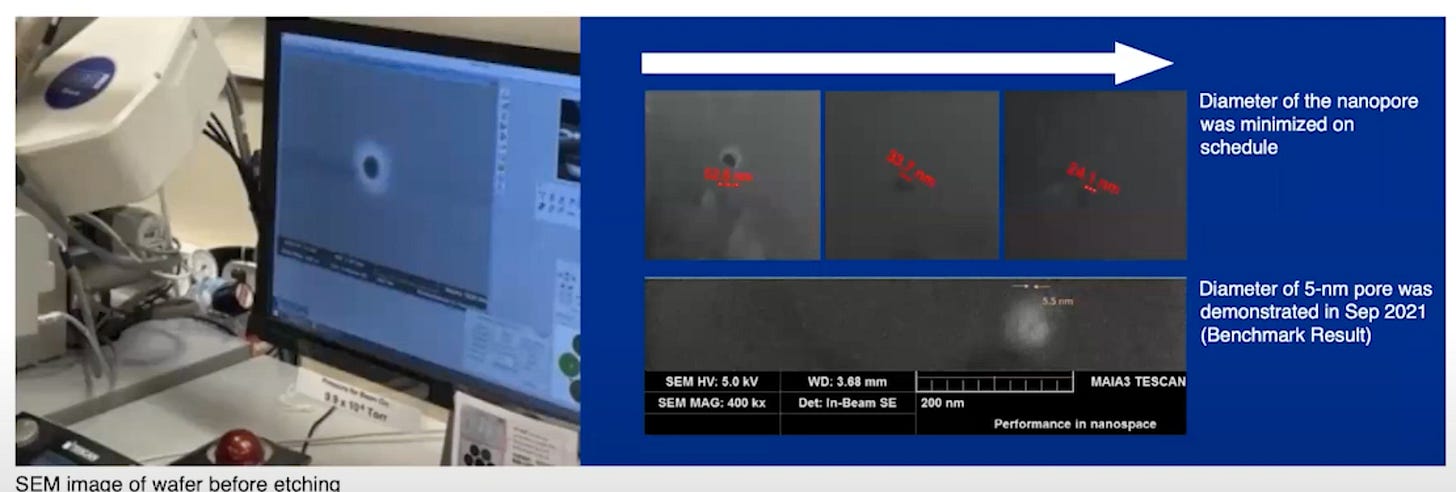
In any case, so far this is all theoretical, as yet Genvida don’t seem to have shown any experimental data. I suspect they are really just getting started.
One of there videos does show some real pores however:
For Genvida, the pore size seems less critical than in other approaches. For the most part the pore just needs to confine the motion of the strand such that it is adjacent to the electrodes.
This brings us to the currently unaddressed problem with the Genvida approach, motion control.
At native speeds DNA translocates at something like a million bases per second.
As I’ve noted elsewhere, 1 picoamp sampled at 1Mhz is ~8 electrons per sample. This means that there are practical limits to how fast you can sense DNA as it translocates through a pore. Their simulations show we need to be able to see 30pA differences between current levels. Practically speaking you’re unlikely to able see these differences using a sample rate of 100KSPS (in fact, Axopatch which they appear to be using will only provide useful data at ~10KSPS).
So to build a sequencer Genvida will need to slow down the translocation to the point where 30 picoamp current differences can be detected. Protein nanopore approaches control the translocation speed enzymatically. It seems possible that this approach could also be applied to a solid state nanopore platform… but perhaps Genvida have other ideas.
However, I suspect we’ll have to wait a while to find out! But as always, I’ll be keeping an eye on them.