NanoMosaic - High Dynamic Range Proteomics
NanoMosaic popped up on my feed, having recently raised $40.8M and stating that their first-gen Proteometics instruments “provides 7+ orders of dynamic range… 384 samples/run in 20 mins”. They say they have installed instruments at a couple of locations.
As always the press releases are light on technical details, and I was interested in digging a little bit deeper.
Nanomosaic appears to be a spinout from the Quan lab at Harvard. I could only find a single patent associated with the company. This patent didn’t describe this sensing approach in much depth. The focus of this patent is the ability to sense over a large range of concentrations (from pg/mL to ug/mL). Their approach uses a combination of single molecule and bulk measurements which is somewhat reminiscent of Quanterix.
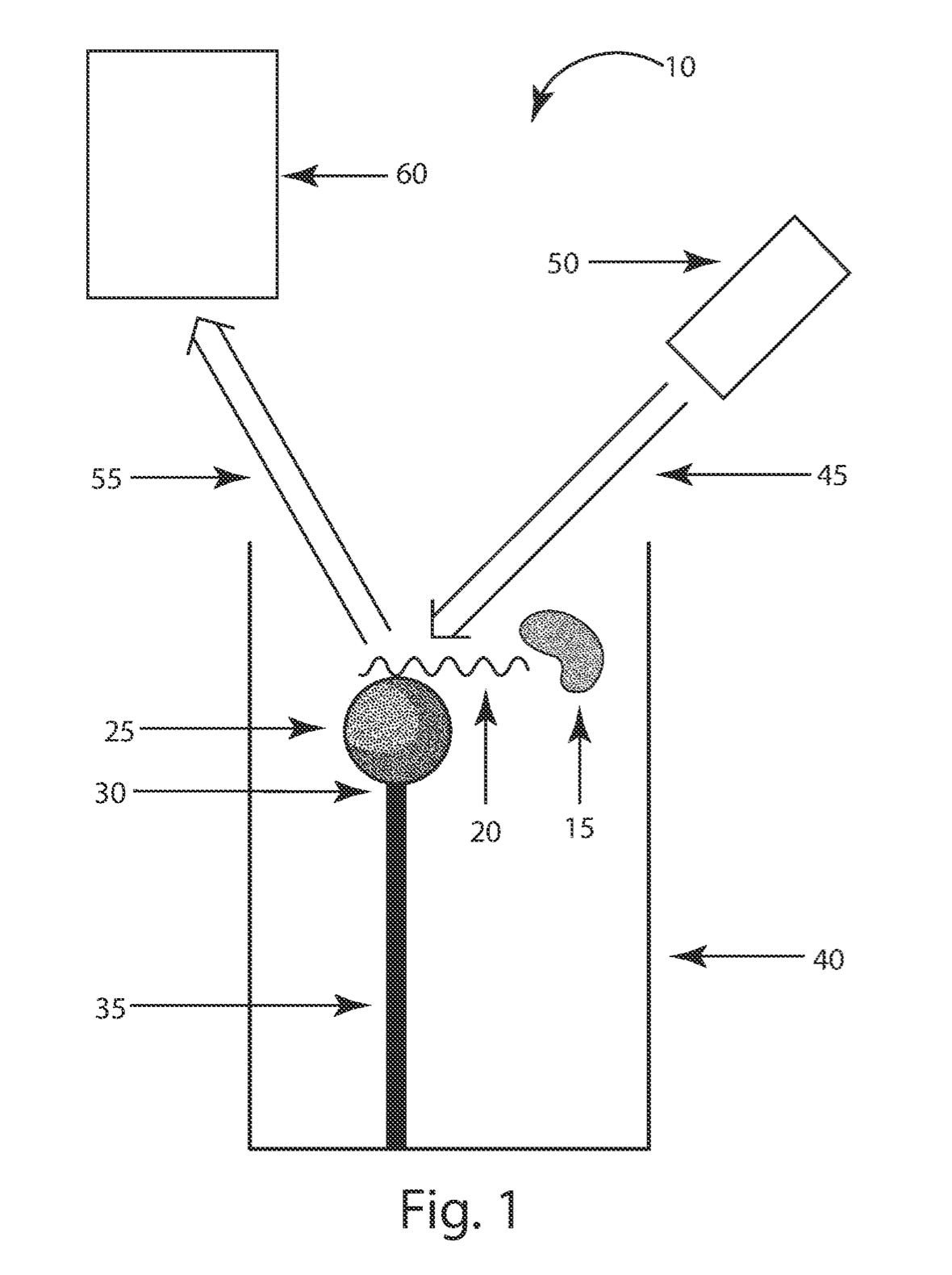
To get a better understanding of the basic sensing approach I look through Quan’s patents. This patent in particular appears to describe a sensing approach that I suspect is used by Nanomosaic, and I assume is part of the “IP licensed from Harvard” mentioned in press releases. In description below, I draw upon figures from both these patents.
To my non-physicist eye, this looks like a nano-antenna, LSPR approach. LamdaGen have a nice video contrasting regular SPR, and LSPR:
The Quan/Nanomosaic approach seems somewhat similar to me. There’s an array of nano-antennas which exhibit resonance under illumination. More simply, nano-antennas will “light up”, or show color-shifts under illumination.
The nano-antennas can be coated with an antibody, and when an antigen binds, this will cause a change in the surface resonance which can be detected (using a relatively cheap camera).
Patents suggests that the effect can be detected both on a single molecule and bulk level. They call their single molecule sensing domain “digital” sensing. So their sensing array is broken don into a digital region, and an analog region:
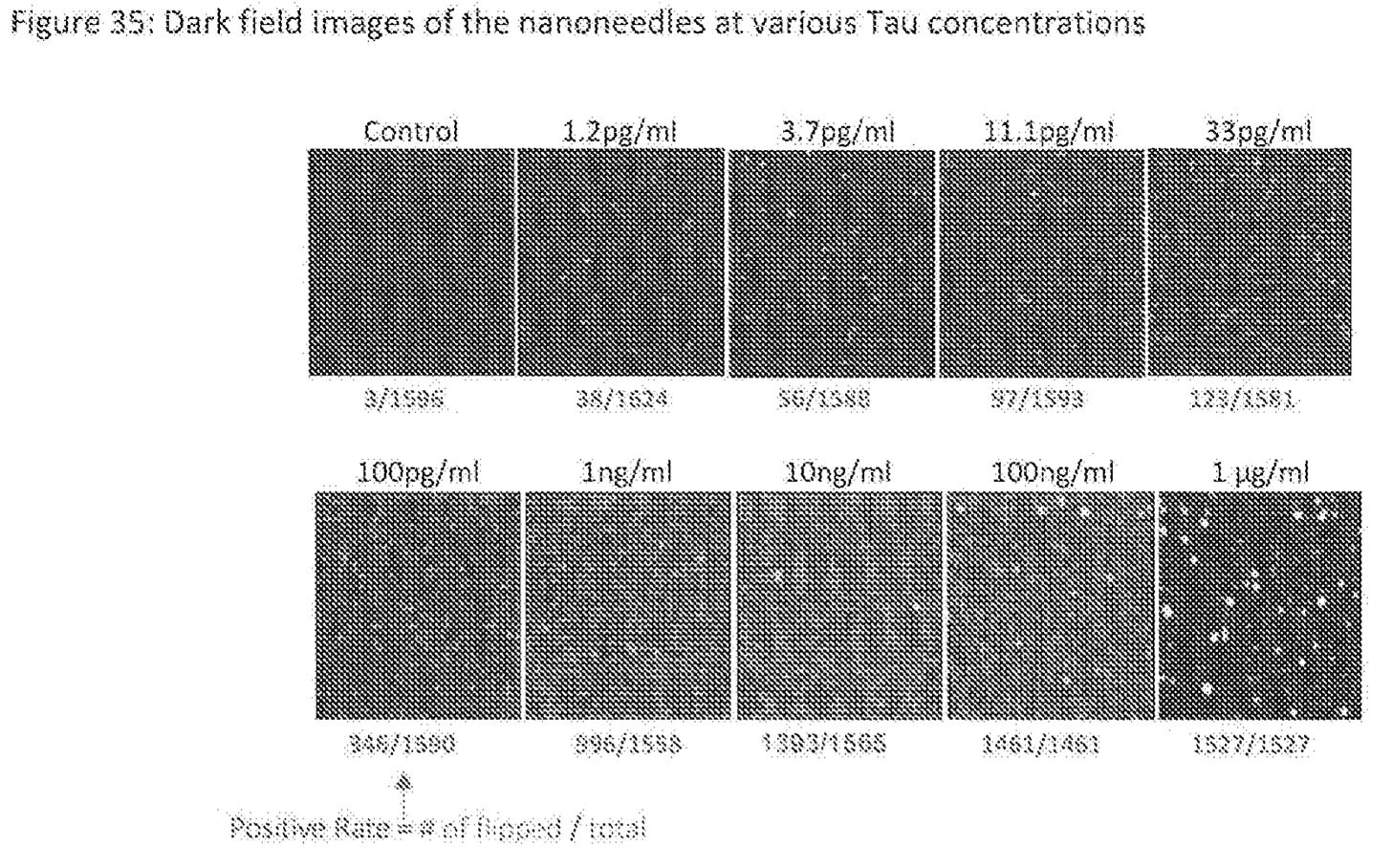
In the digital region nano-antennas are designed such that only a single antigen is likely to bind to a single region/nano-antenna. As such, these exhibit a “flip” from an unbound to a bound state when single molecule binding occurs:
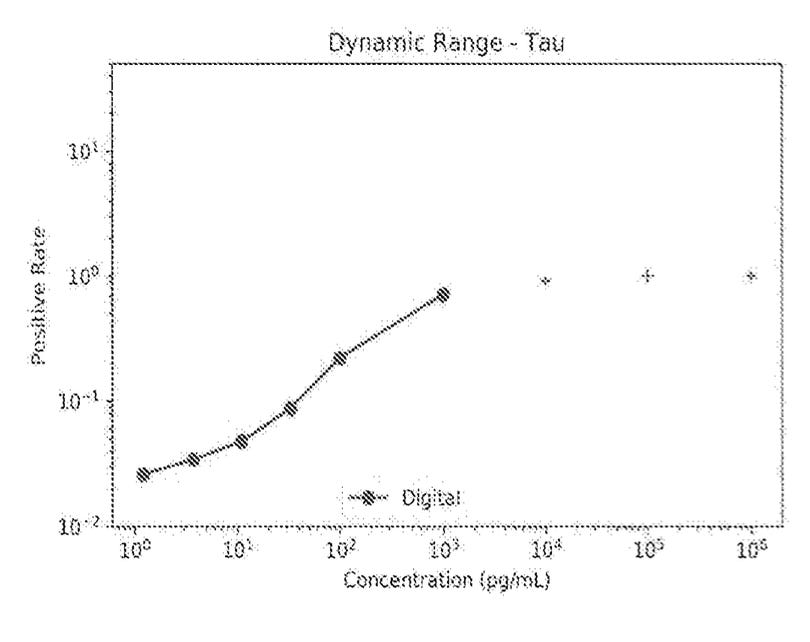
This appears to work well in the pg/ml to ng/ml range. Beyond this, the digital arrays appear to get saturated, and are no longer useful:
The patent also shows digital sensing arrays where detection is in the fg/ml range. This should to let them hit the same sensitivity as Mesoscale/Quanterx.
Based on the above, it looks like the digital arrays saturate at ~1ng/ml. To increase the dynamic range of the platform the chips therefore also contain an analog sensing region. In this region we’re no longer really interested in individual antibodies/nano-antennas. But use larger/denser nano-antenna arrays and look at the bulk color shift:
In this example they’re using a diameter of 95 nm for the digital nano-antennas and up to 130 nm for the nano-antennas in the analog sensing region.
By combining the digital and analog sensing regions they can increase the dynamic range of the platform. In the Tau example they show measurement over 6 logs of concentration on the same chip. This is higher than I’ve seen from Mesoscale and Quanterix.
With examples covering ~1 fg/ml to 1 ug/ml, for the same analyte, they may be able to push this to 9 logs.
The fundamental advantage of the Nanomosaic approach therefore appears to be in providing a large dynamic range. They motivate this with the example of cytokine release syndrome. Suggesting that emerging studies have identified a panel of predictive biomarkers (CRP, ferritin, ΙΕΝγ, IL-6, TNFa and others). And stating that these markers collectively vary in concentration over a large dynamic range. This like a potentially interesting application.
I suspect the instrument itself is also relatively simple/cheap to manufacture. The consumables will obviously require nano-fabrication, and are unlikely to be reusable. As such, I wouldn’t expect the consumables to have a relatively high COGS. So, they’d likely target monitoring (or research) applications (as they suggest with CRS) rather than high volume screening.
I’d be curious to know if other methods (like Quanterix) could be extended in dynamic range while retaining a lower consumables COGS. Recent press releases from Nanomosaic, also suggest they are focusing on aptamers, though I didn’t see any work on this in the patents. In any case, I find the approach technically interesting, and will be keeping an eye out for further developments.