The BioFire FilmArray
Over on twitter someone pointed me in the direction of the Biofire FilmArray platform. I’d seen these devices around, but mostly assumed they were some kind of integrated microarray platform and not looked further. Well, turns out my assumptions were wrong, and the instrument is a little bit more interesting than that.
It’s actually more like the Cepheid GeneXpert (which I’ve discussed a couple of times before). The instrument takes a cartridge, and rather than running a single qPCR reaction can run a number of assays in parallel.
How does it work?
The platform’s key advantage over the Cepheid approach is that you are assay 20+ targets (rather than ~4 on the Cepheid). This is enabled by their integrated “pouch” platform containing freeze died reagents:
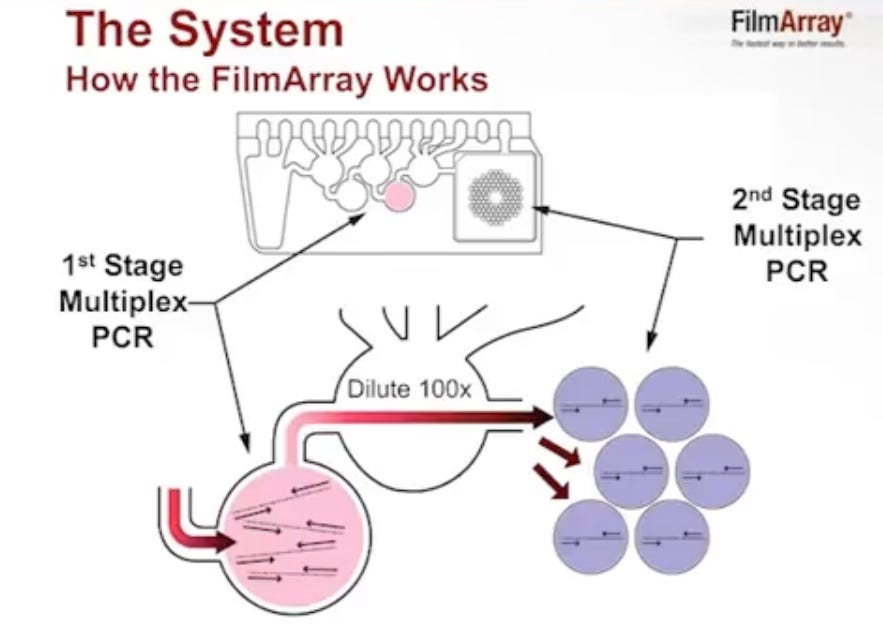
The process is described well in this YouTube video. This is fairly similar to the Cepheid system automating extraction/purification steps. The most significant differences are in the amplification and readout.
The instrument performs a two stage PCR process. In the first stage, the instrument essentially just amplifies all targets in a single reaction volume. This is what you’d usually do in a multiplex qPCR platform (like the Cepheid instruments), amplify the DNA in one tube and probe with different labels. For example in COVID-19, this is how you can assay different targets in a single reaction.
But you can generally only really squeeze in 6 targets that way (there’s too much dye overlap beyond this).
So the FilmArray only uses this first stage PCR reaction (containing up to 90 primers) to amplify material and runs for only ~20 cycles. After this they add a second stage PCR mix, and move the material over to the array.
Each well in the array contains a second set of primers and an intercalating dye. Patents suggest they run at least 32 cycles in this second stage.
From what I’ve seen, seeing amplification (Ct) at cycle 25 is typical. So in Biofire’s case, it doesn’t seem like the first stage amplification is reducing the number of cycles required in the second stage PCR, and in total the instrument probably runs ~60 amplification cycles across both stages.
In addition to qPCR the instrument also perform a melting point analysis. Increasing the temperature until the double stranded DNA dissociates, and fluorescence from the intercalating dye disappears. Melting temperature varies with sequence composition. So they can use this as an additional control that the correct target as been amplified and that the primers have not mis-hybridized and amplified the wrong target.
Comparison with Cepheid
All things being equal, the FilmArray platform would obviously be a better option than the Cepheid, 20+ targets? Great! Well, the first obvious issue is cost. From what I could find the FilmArray consumables cost $109 to $210. BioMérieux financial results suggest that they operate at ~50% margins. So it seems likely that the COGS on these pouches is >$50. In contrast the Cepheid cartridges probably cost ~$3 to make.
Beyond this, there are a few potential technological issues. As far as I can tell, the FilmArray instruments don’t report Ct values. Based on the technological approach, it seems unlikely that FilmArray and regular qPCR Ct values are directly comparable. The readout really operates much more like an Endpoint PCR platform than qPCR.
There are some excellent graphs which show the issue with endpoint PCR. Essentially, you can’t use the endpoint value to easily estimate viral load or to provide much more than a binary yes/no output. Whereas with qPCR, you get reasonably quantitive information on the viral load based on the Ct value.
I decided to try this out for myself as I’ve been playing with a SmartCycler recently and yes, it’s pretty clear that in my unskilled hands, the Endpoint values vary a lot more than the Ct on technical replicates (which isn’t really saying much, but was a fun experiment):
As a yes/no diagnostic platform reports suggest that the FilmArray performance is similar to Cepheid however.
Summary
Overall, the FilmArray is kind of a neat platform, much like the Cepheid GeneXpert they have integrated sample prep and detection in to a “sample-to-answer” platform. But in their case, they can assay >20 targets with a single cartridge.
However, they don’t have a clear cost advantage. We could probably assay 48 targets for the same COGS using the Cepheid approach (16 cartridges at $3, each assaying 3 targets).
This, coupled with the potentially less quantitive data from the FilmArray means that the FilmArray isn’t a clear win over using a Cepheid.
A sequencing based Cepheid-like platform however, well… that would surely be a clear win.